We are motivated by a strong and sincere willingness to drive continuous improvement and renewal within ADME/PK science. We believe that a technology shift and significant quality improvements are within reach. Together we form a group of highly merited scientists with a long experience from the pharmaceutical industry, university and IT-development.
ADME/PK-prediction software with predictive power
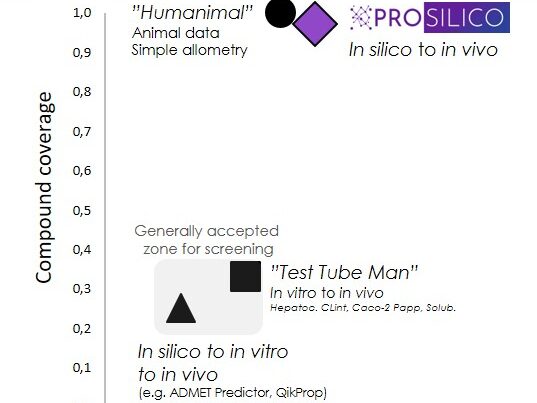
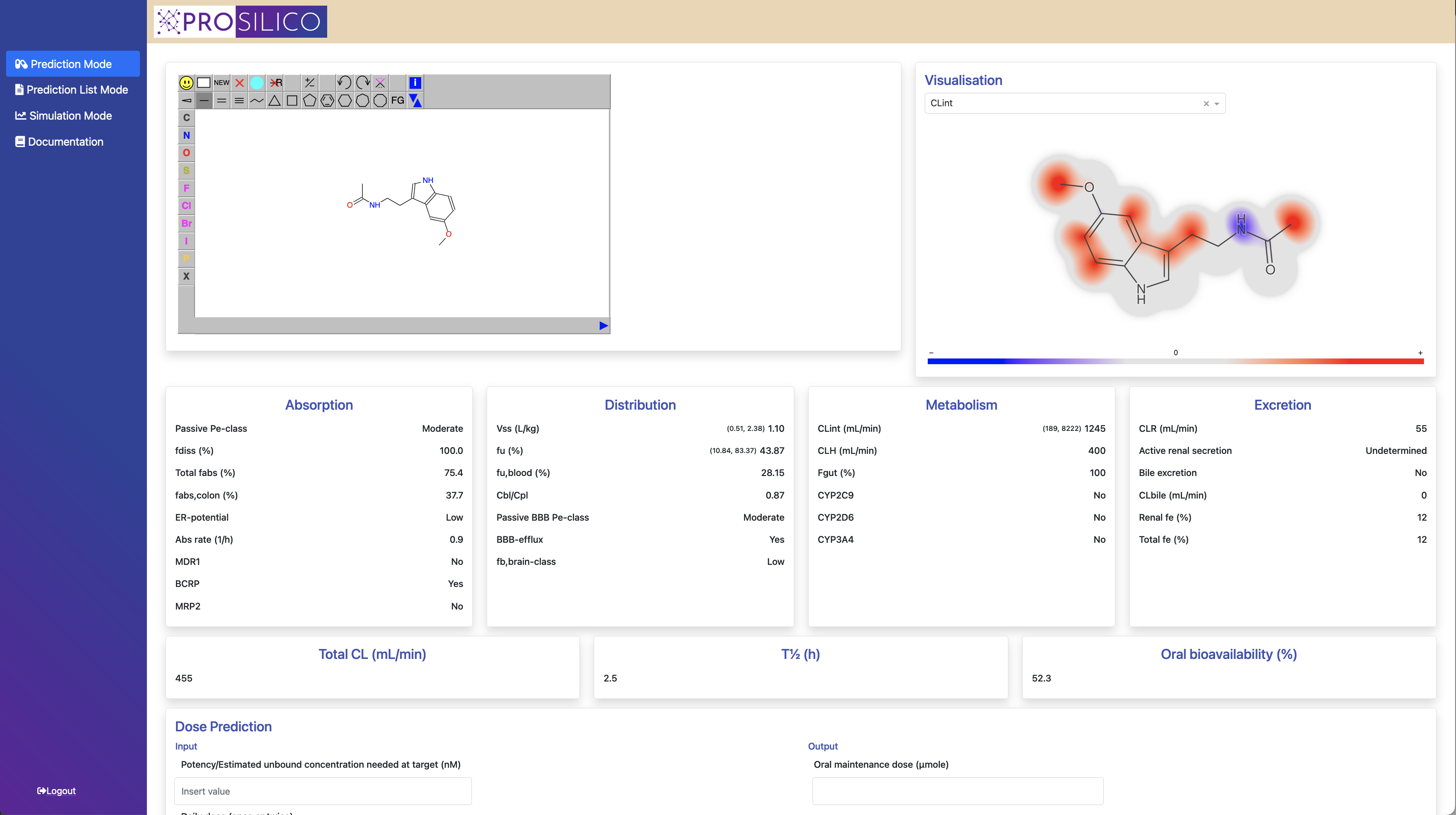
Our software ANDROMEDA by Prosilico produces high quality in silico estimates of human clinical ADME/PK of modern drug candidates (for which standard laboratory methods have limited applicability) at a low cost. Our approach is firmly based on the assumption that clinical data is the best and most relevant source of human ADME/PK information.
The software has been carefully validated in a multitude of ways. We have also performed successful benchmarking vs alternative methods (in vitro and animal models). Median prediction errors are generally within 2- to 3-fold. Scientific publications and White paper are available to support our claims.
Make you feel good about it
You can rely on the fact that the human clinical ADME/PK parameters you get are the result of validated and relevant methods. The predictive power increases the likelihood that your compounds will have the desired ADME/PK properties in man and that clinical studies will be safe and planned for success. As a bonus you will also help to avoid unnecessary experiments in animals and reduce environmental emissions.


The latest news and publications